Spotlight
Highlights at BIO Asia–Taiwan 2025
At BIO Asia–Taiwan 2025, ITRI unveiled several groundbreaking technology innovations, demonstrating its strong capabilities in biomedical R&D. Highlights included next-generation long-acting vaccine adjuvants, novel therapies for cancer and eye diseases, and AI-driven platforms for diagnostics and data analysis.
Eric Y. Chuang, Vice President and General Director of ITRI’s Biomedical Technology and Device Research Laboratories, emphasized that ITRI has been actively exploring innovation in precision medicine and cancer treatment. “From nucleic acid drugs targeting solid tumors to advanced exosome-based diagnostics, we’ve been working to make therapeutic solutions and detection processes smarter and more accurate. While fueling the growth of the biomedical industry, we aim to improve public health and enhance well-being,” he said.
Key highlights from this year’s exhibition:
CD40 Ligand Ribonucleic Acid Vaccine Adjuvant for Long-Lasting Immunity
Subunit and peptide vaccines with conventional adjuvants often fail to induce long-lasting immune responses and require frequent booster shots. While current anti-CD40 antibody adjuvants effectively activate the immune system, they can cause liver toxicity due to excessive immune stimulation. To address this, ITRI developed a hexameric CD40 ligand ribonucleic acid vaccine adjuvant that elicits strong antibody and T cell responses, promotes immune memory, and reduces cytotoxicity. In animal studies, the hexameric CD40 ligand mRNA showed a twofold greater inhibition of brain tumor growth in mice compared to another product in Phase III clinical trials and provided durable protection against lymphoma for over six months.
This innovation enhances vaccine durability, reduces the need for booster doses, and minimizes side effects caused by conventional anti-CD40 antibody adjuvants. ITRI is currently collaborating with Vectorite Biomedical Inc. to optimize immune cell expansion protocols, with the aim of applying this platform to both infectious diseases and cancer immunotherapy.
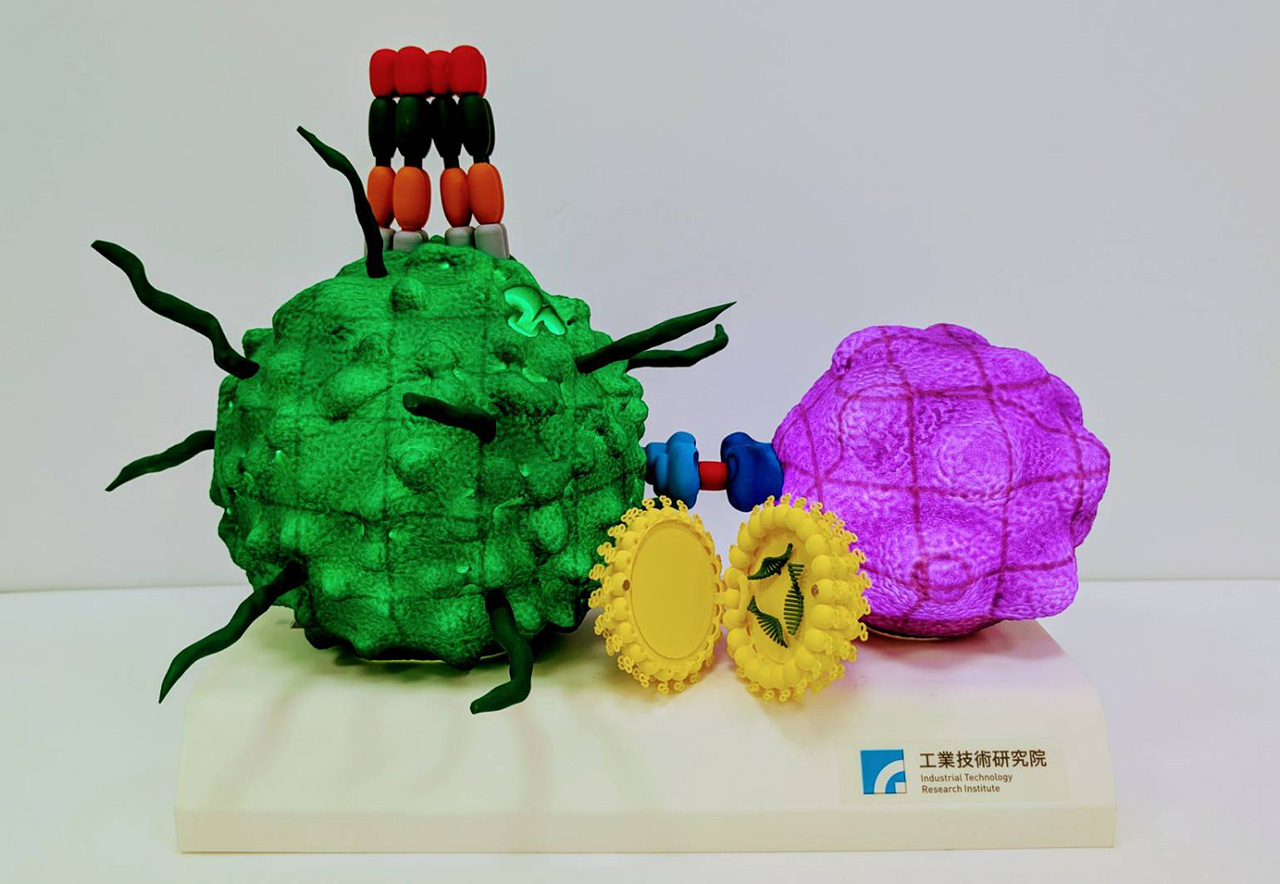
The CD40 ligand mRNA vaccine adjuvant can elicit a long-lasting cytotoxic T cell memory response.
In Vivo Delivery of mRNA Encoding CAR to Macrophages for Solid Tumor Therapy
ITRI has developed a novel chimeric antigen receptor macrophage (CAR-M) cell therapy for solid tumors by the intravenous administration of lipid nanoparticles (LNPs) encapsulating mRNAs that encode a tumor-associated antigen (TAA)–targeted CAR and an M1 macrophage–polarizing factor. This innovation enables the activation and direct expression of CARs in macrophages within the body, eliminating the need for ex vivo cell engineering. It also improves tumor recognition accuracy, enhances macrophage tumor-killing activity, and reduces the risk of off-target effects or adverse reactions.
Designed as an off-the-shelf immunotherapy, this technology lowers manufacturing costs and enables a broader anti-tumor immune response compared to traditional cell therapies. In a syngeneic mouse model, intravenous administration of CAR mRNA-LNP (encoding M1-polarizing and TAA-specific CAR) effectively suppressed hepatic tumor metastasis, reducing the liver metastasis rate from 85% to 25%, without the need for immune checkpoint blockade.
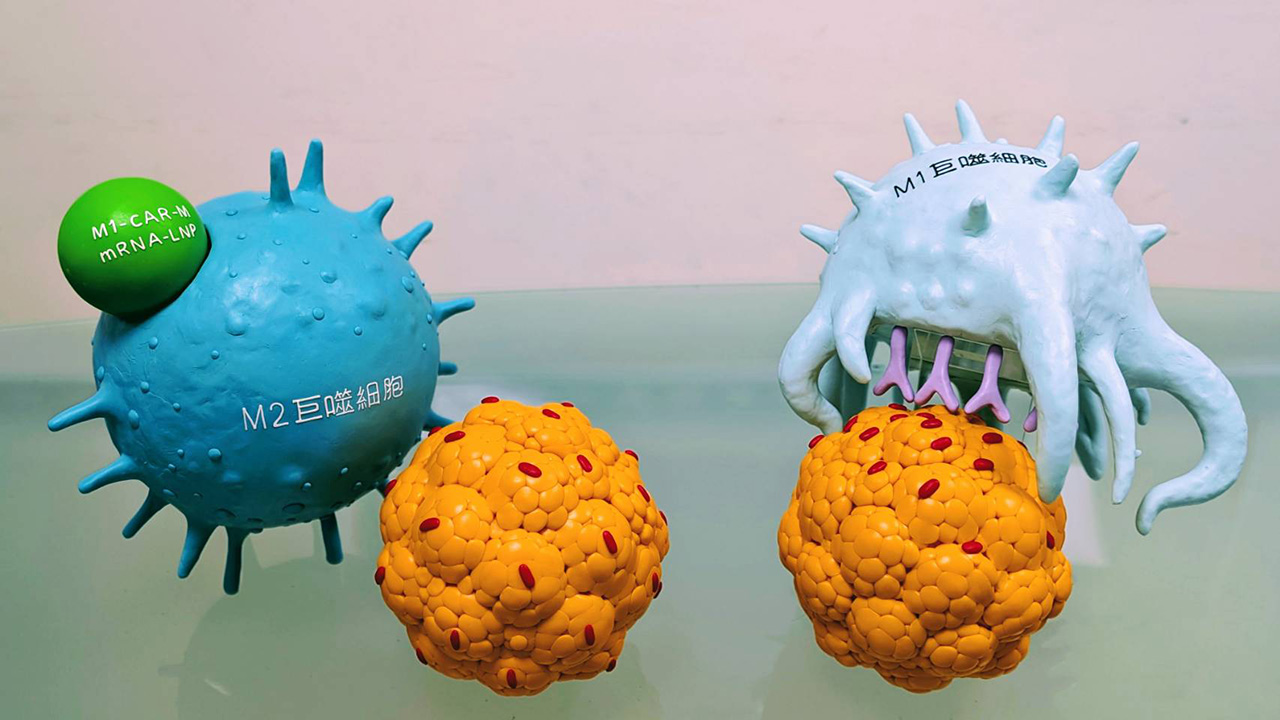
Liver-targeting lipid nanoparticles with M1 factors and CAR mRNA can suppress tumor growth and metastasis.
An Eye Drop Product for Dry Age-Related Macular Degeneration (AMD) Treatment
Posterior eye diseases are commonly treated with intravitreal injections, which carry risks of infection and often result in poor patient compliance. ITRI’s proprietary eye drop product, formulated with FDA-approved excipients, provides a non-invasive, self-administration option for managing these conditions. Utilizing Coordination Supramolecular Complex (CSC) technology, the eye drop formulation effectively delivers drugs to posterior ocular tissues.
In preclinical studies, this eye drop was well-tolerated over a three-month dosing period and demonstrated superior efficacy compared to the clinical benchmark drug in preserving photoreceptor integrity and reducing sub-retinal pigment epithelium deposits, supporting strong clinical potential. ITRI is actively collaborating with industry partners to co-develop products and pursue licensing opportunities, strengthening ophthalmic innovation and global competitiveness.
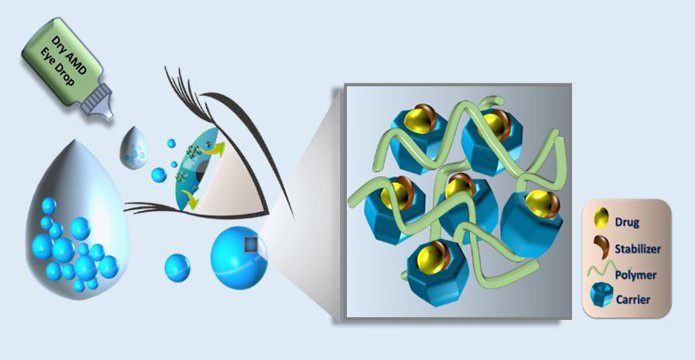
ITRI has developed an eye drop product for dry AMD treatment.
Tumor-Derived Exosomes Enrichment and Detection Platform
Developing exosome biomarkers for liquid biopsy is a complex, time-consuming process that typically takes 5 to 10 years. ITRI has introduced a high-precision tumor exosome detection platform that utilizes machine learning algorithms for big data analysis along with magnetic bead-based enrichment technology.
This system enhances specificity and doubles detection sensitivity, accelerating biomarker discovery by 1 to 2 years. A monitoring kit for triple-negative breast cancer recurrence has been developed, achieving a detection accuracy of over 80%. The technology enables early cancer detection, creating new opportunities in the exosome diagnostics market.
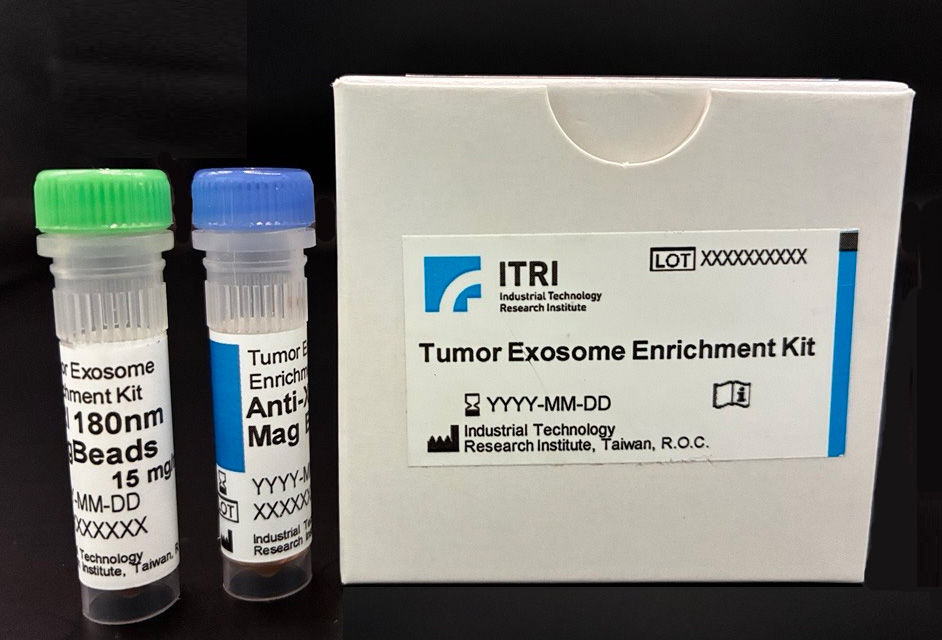
A tumor exosome enrichment kit has been developed for breast cancer recurrence detection.
One-Stop Exosome Isolation and Characterization Services
To support the development of exosome-based products, ITRI has established a liter-scale exosome isolation platform that combines tangential flow filtration (TFF) and size-exclusion chromatography (SEC) with specialized surface treatments. The process takes less than 2.5 hours and achieves a 10-20% higher recovery and purity than leading commercial systems.

Specialized TFF and SEC columns enabling rapid, high-purity exosome isolation.
ITRI’s platform offers a comprehensive multi-omics analysis service to assess exosome quality, identify key bioactive components, and evaluate their functionality. This integrated, customized service supports large-volume sample processing, routine testing, characterization, and quality standard development, empowering industry partners to reduce product development time and costs.